Virotrac™ Technology
Larad’s Virotrac vaccines are produced using genetic engineering (cloning and protein expression) in the baculovirus system to create viral proteins capable of self-assembling to form virus-like particles (VLPs).
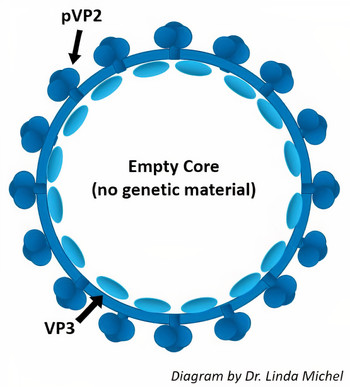
VLPs are structurally analogous to viruses and induce a strong immune response but contain no genetic material and cannot cause disease. Platform vaccines made from VLPs can be rapidly re-engineered to keep pace with mutating field viruses, opposed to conventional vaccines that gradually become less effective over time. Larad's first VLPs, developed for Infectious Bursal Disease Virus, use capsid proteins pVP2 and VP3.
The baculovirus system has shown to be exceptional in creating consistent, quality particles that require minimal downstream processing, reducing the cost of production and the time needed to process the materials into a final product. Baculovirus does not present a health hazard for humans or animals and can be inactivated using several simple techniques, so it has little to no impact on the final product.
Our Virotrac vaccines are considered platform vaccines by the USDA Center for Veterinary Biologics (CVB). Platform Vaccines are not Autogenous vaccines, which are given more stringent regulations by the CVB. Autogenous vaccines are required to be prepared using an isolated pathogen from the farm where the vaccine is intended to be used, and vaccine batches are only approved for use over a limited period of time. The CVB approved Platform Vaccines for any geographic location in the US and there is no limit on the length of time they can be used (VS Memorandum #800.213).
Larad's VLP vaccines also eliminate the need to isolate and propagate the pathogens prior to vaccine production, which significantly reduces the time needed to produce a safe, custom product.
